matterlogoboss
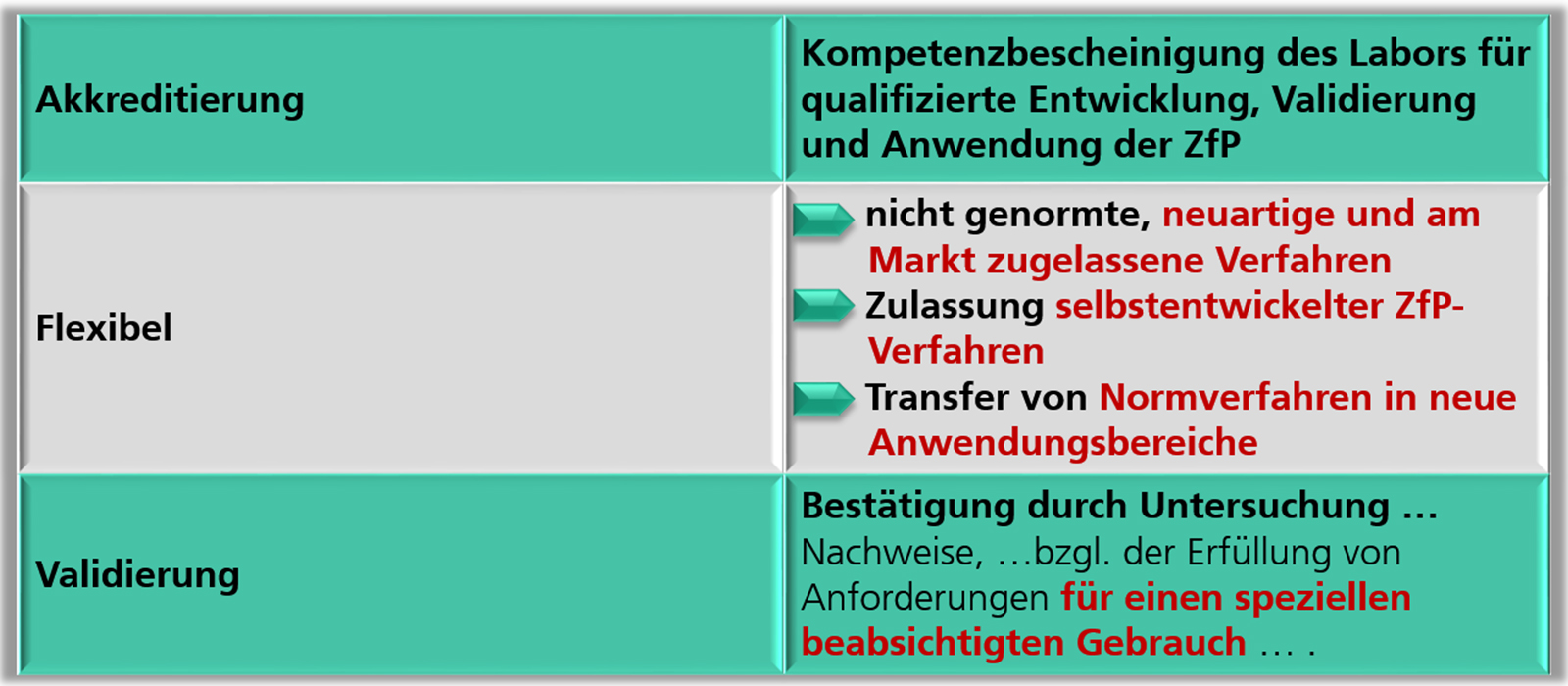
Akkreditierung Nach Din En Iso Iec 17025
News Successful re-accreditation of the quality management system according to ISO/IEC 17025 The new scope of testing comprises approx. 300 accredited international, national and in-house test methodsEndoLab® is pleased to announce that the Deutsche Akkreditierungsstelle GmbH (German Accreditation Body, DAkkS) confirmed that our testing laboratory fulfills the requirements of the ISO/IEC quality management standard and is authorized to conduct tests in the field of physical testing of medical devices as well as of physical and physical-chemical testing of non-active implants. Our new scope attests our expertise to perform tests according to almost 300 accredited international (ISO/ASTM), national (DIN) and in-house test methods. Please find our new certificate of the re-accreditation and corresponding scope at.
Information on Registered Substances comes from registration dossiers which have been assigned a registration number. The assignment of a registration number does however not guarantee that the information in the dossier is correct or that the dossier is compliant with Regulation (EC) No 1907/2006 (the REACH Regulation).

Iso Iec 17025 2017 Pdf
This information has not been reviewed or verified by the Agency or any other authority. The content is subject to change without prior notice. Reproduction or further distribution of this information may be subject to copyright protection. Use of the information without obtaining the permission from the owner(s) of the respective information might violate the rights of the owner. Fusionfall full version.